Potential for Suspension of Dynamic Sensing Algorithm
Aurora EV-ICD™ (DVEA3E4) and Clinical EV-ICD (DVEX3E4)
Original Date of Communication:
October 2025
View specific models that this applies to
- The manufacturing change and SmartSync release mentioned below are based on regulatory approvals. Check with your Medtronic Representative for approval status in your geography.
- The serial number look up does not identify devices that have been updated via SmartSync. A CareLink report is available to assist with identifying devices that require the update, contact your Medtronic representative to obtain a copy of this report.
ORIGINAL COMMUNICATION - OCTOBER 2025
In a subset of Aurora EV-ICD™ (DVEA3E4) and Clinical EV-ICD (DVEX3E4) devices, there is a potential for delayed time to high-voltage therapy should a rare sequence of events occur. A device update via CareLink™ SmartSync™ Device manager is available to eliminate this potential delay. Through 2 October 2025, six (6) events (delay of 2-17 seconds) were observed among approximately 4,900 implanted devices worldwide (~0.12%). Five of the six devices experienced this behavior during a controlled defibrillation threshold test. While not observed clinically, a delay in HV therapy may impact defibrillation efficacy. There have been zero reports of permanent harm or death due to this behavior.
The root cause of this behavior is related to the EV-ICD dynamic sensing algorithm becoming static if a charge-end occurs while the device is processing a sensed event. This will set the sensitivity to 53% of the prior R-wave amplitude. A subsequent R-wave amplitude that exceeds the static 53% level will restore automatic sensitivity operation and allow R-wave synchronization for therapy delivery. See Appendix A for additional details.
Medtronic has implemented manufacturing changes, and devices manufactured with this update are not susceptible to this delay. In previously distributed devices, a device interrogation with the CareLink™ SmartSync™ Aurora EV-ICD™ application (D00U025) on CareLink SmartSync Device Managers will resolve the potential for this behavior (see Appendix B).
Individual devices susceptible to this behavior can be identified via search/look-up on the Medtronic Product Performance Report Website (productperformance.medtronic.com).
PATIENT MANAGEMENT RECOMMENDATIONS FOR PREVIOUSLY DISTRIBUTED DEVICES:
Medtronic acknowledges that each patient requires unique clinical considerations. Based on internal investigation and consultation with our Independent Physician Quality Panel, Medtronic provides the following guidance:
- Prophylactic device replacement is NOT recommended.
- Schedule an in-clinic SmartSync interrogation to receive the software update, with the intent for all patients to receive the update within the next 3-6 months.
For patients remotely followed via CareLink, your Medtronic representative can provide a report to assist with identifying patients that require this in-person update. You may contact your local representative to obtain an updated copy of the report at any time.
APPENDIX A
TECHNICAL DETAILS
The Aurora EV-ICD dynamic sensing algorithm is unique due to the implant location within the substernal space. Figure 1 below illustrates how the device sets sensitivity beat-by-beat based on R-wave measurement of the rectified and filtered EGM.
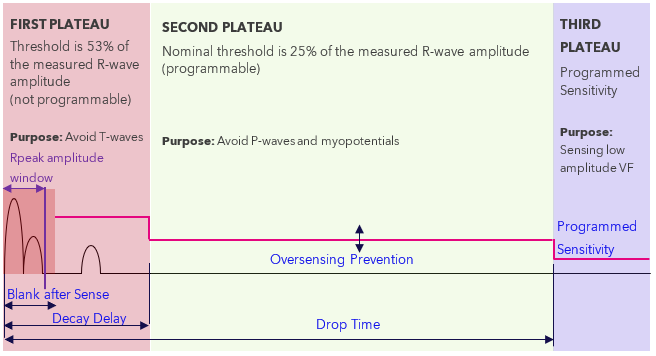
Figure 1 - Operation of Sensing Thresholds - The first plateau duration (“Decay Delay”) is nominally 360 ms, the second plateau duration (“Drop Time”) is nominally 1500 ms, and the third plateau lasts until another sensed beat. R-wave amplitude measurements take place during “Rpeak amplitude window” shown above, nominally 128.75 ms (not programmable).
The dynamic sensing algorithm may become suspended at the first plateau if the following sequence of events occurs:
- Charge-end occurs within 128.75ms of the prior sensed beat, thus interrupting the in-progress R-wave measurement-suspension of the dynamic sensing algorithm occurs and the sensitivity plateau becomes static at 53%.
- The sensitivity plateau will remain at 53% until a subsequent R-wave amplitude exceeds this value. In the case of a significant drop in R-wave amplitudes, delay in HV therapy delivery can occur.
While not observed clinically, a delay in HV therapy may impact defibrillation efficacy and result in harm related to failure to terminate an arrhythmia.
This behavior applies only to non-committed shocks. This includes cardioversion (CV) therapies in the ventricular tachycardia (VT), fast ventricular tachycardia (FVT) zones, and the first shock in the ventricular fibrillation (VF) zone.
A Medtronic white paper, Suspension of the Dynamic Sensing Algorithm: Determining the Potential Occurrence, Duration, and Patient Impacts of Delays to Therapy, with additional technical details is available from your Medtronic representative if desired.
APPENDIX B
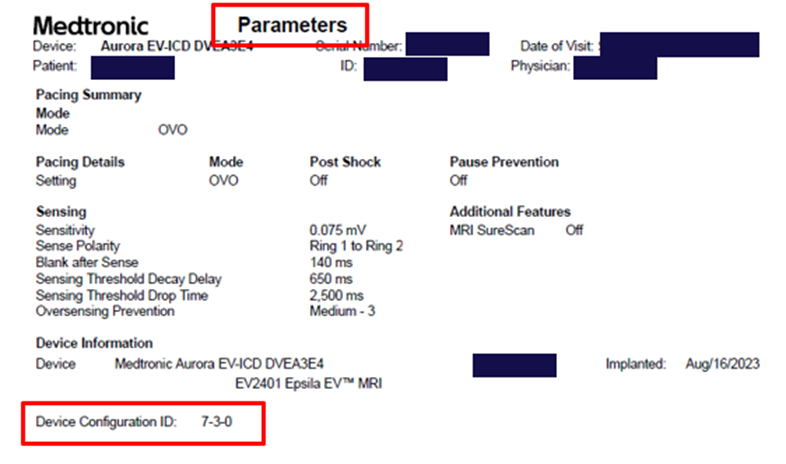
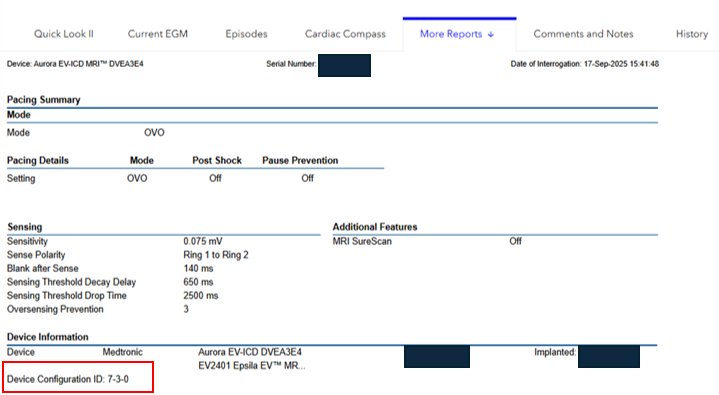
To identify if a patient’s device has successfully received the update, view the displayed Configuration ID and confirm the first number in the sequence as indicated below:
- Clinical device (DVEX3E4) configuration ID is 6-1-0 or greater
- Aurora EV-ICD (DVEA3E4) is 7-3-0 or greater
The Device Configuration ID can be found under the “Device Information” section of the SmartSync Parameters Report, or for CareLink patients, under the Transmission Details page by selecting
To identify if a device was manufactured with the update, look for version 02 or higher listed underneath the barcode:

Specific Models This Applies To
Methods for Estimating
Contact Us
Worldwide Contact Information