Product Education Brief: Boston Scientific Lead Advisory
Medtronic ICDs and CRT-Ds
Original Date of Communication:
October 2025
View specific models that this applies to
Applicable Product:
Single coil (SC) and dual coil (DC) RELIANCE™ G/SG defibrillation leads manufactured by Boston Scientific Corporation (BSC) from 2002 to 2021 with GORE™ expanded polytetrafluoroethylene (ePTFE) coil treatment connected to Medtronic ICD/CRT-D systems
Executive Summary
- Medtronic devices are NOT at risk of a BSC Code-1005 equivalent fault and its associated constraints on high-voltage therapy delivery, irrespective of shock polarity.
- Medtronic does not recommend reprogramming high voltage therapy polarity of Medtronic devices connected to recalled BSC leads.
- The BSC recommendation to consider lead replacement also applies to leads attached to Medtronic devices.
Content
This Product Education Brief leverages information in published literature, BSC’s July 2025 Field Correction Action (FCA) letter*, and Medtronic data analysis to date to review:
- Reason for the advisory on BSC GORE™ ePTFE RELIANCE™ defibrillation leads
- BSC risk-mitigation recommendations for BSC devices
- Comparison of Medtronic device behavior to BSC device behavior when attached to BSC leads on advisory
- Therapy efficacy/success analysis on Medtronic devices with BSC ePTFE leads
-
Medtronic position on the BSC advisory and Medtronic devices
- Medtronic labeling for therapy polarity remains appropriate for Medtronic devices with BSC advisory leads
Reason for BSC Recall of ePTFE RELIANCE leads*
The association of calcified defibrillation lead coil(s) with a pattern of gradually rising Low Voltage Shock Impedance (LVSI) measurements has been reported to BSC and described in several publications (listed in the BSC advisory letter*). The chronic calcification process originates in the ePTFE membrane coating the defibrillation coils. This calcification phenomenon can biologically encapsulate and electrically insulate the defibrillation lead coil(s), thus potentially reducing the amount of energy delivered and increasing the chances of unsuccessful defibrillation of ventricular tachyarrhythmias. The BSC advisory letter did not provide mitigations to reduce the likelihood of lead calcification. At the time of the advisory letter, FDA disclosed that BSC reported 16 patient deaths, and 386 serious patient injuries related to the lead.
BSC’s Risk Mitigation for BSC Devices
The July 2025 BSC advisory letter provides the following risk mitigation recommendations for BSC leads attached to BSC devices:
“If an ePTFE lead is experiencing a gradually rising LVSI that exceeds 90Ω for a SC or 70Ω for a DC lead [on a BSC device], the risk of compromised shock efficacy can be mitigated by programming all shocks to maximum energy and shock polarity to Initial (RV-) in those patients whose [BSC] devices are not already programmed in this manner. For ePTFE leads with a gradually rising LVSI, there is a 4.5x higher likelihood of generating a EC-1005 in Reversed (RV+) polarity compared to Initial (RV-) polarity [on a BSC device]”*
Code-1005 indicates that a high-voltage energy delivery may have been truncated. Important points regarding the BSC recommendations:
- Medtronic devices are at ZERO risk of a Code-1005 equivalent fault and its associated constraints on high voltage therapy delivery, irrespective of shock polarity (see next section for details).
- The BSC shock-polarity recommendation reduces, but does not eliminate, the potential for triggering Code-1005 in leads with gradually rising impedances.
- For BSC leads demonstrating LVSI ≥ 150ohms, consider lead replacement.
BSC Code-1005 functionality is unique to BSC devices
With respect to the behavior described in the July 2025 BSC advisory, conditions that contribute to Code-1005 include the following:
- As impedance rises in a calcified lead, the pulse width of a high-voltage shock waveform will extend. This is true of all fixed-tilt waveform systems.†
- The BSC first-phase shock delivery in a biphasic waveform is limited to a 20ms pulse width. If the first phase of a BSC shock is not completed within 20ms, the “...bi-phasic waveform is truncated and a monophasic shock is delivered, potentially reducing shock efficacy.”*
The shock-polarity recommendations in the BSC advisory letter provide a path to temporarily reduce the risk of triggering Code-1005 in the BSC system. The statement, “4.5x higher likelihood of generating a Code-1005 in Reversed (RV+) polarity compared to Initial (RV-) polarity”* is not applicable to Medtronic devices.
Medtronic High Voltage Delivery
Differences in Medtronic device design are not accounted for in the BSC advisory recommendation. Specifically:
-
Medtronic devices use a 50% first-phase tilt (versus a 60% first phase tilt in BSC devices).
- This means the first phase of energy delivery needs to unload a lower percentage of total energy in Medtronic devices before the first-phase waveform timeout is reached than in the BSC system.
-
Medtronic devices allow a 25.25ms pulse width for the first phase of waveform delivery. Due to differences in tilt and capacitance in Medtronic devices, waveform timeout will not typically occur until high-voltage impedances exceed ~200-250ohms.
- Per the BSC advisory letter, "If HVSI exceeds 145 ohms, BSC defibrillators, by design, limit shock duration of the first shock phase to 20ms. If this occurs, the shock’s bi-phasic waveform is truncated and a monophasic shock is delivered, potentially reducing shock efficacy".†
-
In Medtronic devices, if the entire first-phase energy has not been delivered within 25.25ms, the system will proceed to the second phase of energy delivery at the current voltage and deliver in the opposite polarity.
- The BSC devices will truncate energy delivery at 20ms, resulting in a monophasic, truncated energy delivery when BSC Code-1005 conditions are met.
For these reasons, the BSC advisory polarity programming recommendations are not applicable to Medtronic devices.
Therapy Efficacy Analysis - Medtronic Devices with BSC ePTFE leads
Medtronic analyzed high-voltage events with BSC ePTFE leads attached to Medtronic devices in CareLink data. Every shock was assessed by Medtronic subject-matter experts for high-voltage pulse width, high-voltage impedance, and total voltage delivered to determine whether the device performed as expected. Leads with atypical performance were manually adjudicated for first shock success and episode success.
The manual adjudication of each therapy shows that Medtronic devices successfully delivered full-energy, biphasic, high-voltage therapies with BSC ePTFE leads in the Medtronic nominal/recommended B>AX polarity for all therapies regardless of the LVSI. Image 1 below demonstrates that Medtronic therapy success rates for these events are consistent with historical, expected performance. Of note, when coil impedances rose above the 70/90 ohm normal range, Medtronic observed an increased potential for abnormal therapy pulse widths. Medtronic devices will not truncate therapy due to an abnormal pulse width, but the abnormal pulse width signals that the lead may be compromised and may not perform as expected.
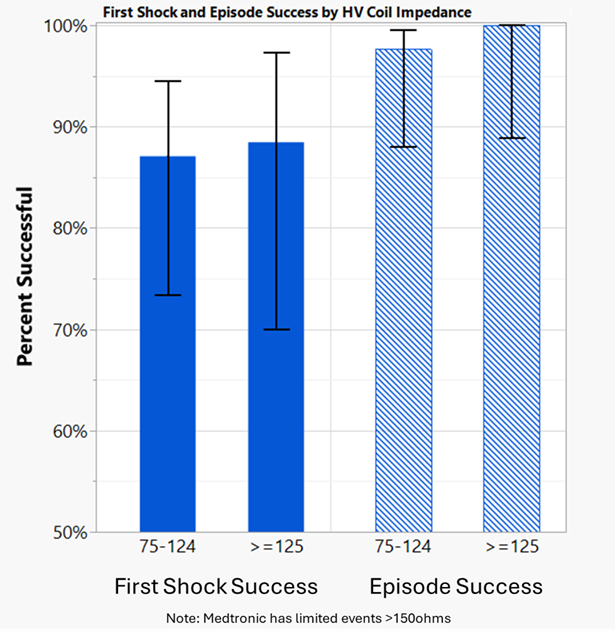
Image 1:First shock and episode success rates in B>AX polarity for Medtronic devices attached to ePTFE BSC single and dual coil leads with evidence of coil calcification
Summary - Medtronic position regarding BSC recalled leads connected to Medtronic devices
-
Medtronic devices are NOT at risk of a BSC Code-1005 equivalent fault and its associated constraints on high-voltage therapy delivery, irrespective of shock polarity.
- This difference in risk is due to a combination of Medtronic waveform tilt, capacitance, leading edge voltage, and algorithms controlling high-voltage shock delivery.
- In Medtronic devices, first shock and episodic success remains consistent with historical performance across HVSI impedance ranges described in the BSC communication with the nominal/recommended polarity.
-
Medtronic does not recommend reprogramming high voltage therapy polarity of Medtronic devices connected to recalled BSC leads. Medtronic devices that include labeling recommendations for shock polarity will display a pop-up warning if a non-nominal polarity setting is selected. These devices should not be reprogrammed in an attempt to replicate the recommended programming for BSC devices, as data does not support this action in Medtronic devices.
- Medtronic therapy success (Image 1 above) is not limited by the shock polarity bias described in the BSC advisory letter when attached to BSC calcified leads.
-
Table 1 of the BSC advisory includes lead-replacement considerations under defined conditions. This BSC recommendation also applies to leads attached to Medtronic devices.
-
When coil impedances rise above the 70/90 ohm normal range, there is increased potential for abnormal pulse widths. Abnormal pulse widths signal that the lead may not perform as expected.
- In BSC devices, “HV impedance > 145 Ohms can alter the waveform of defibrillation and result in ineffective monophasic shocks.”†
- “Endotak Reliance defibrillation leads appear to be prone to shocking coil and/or distal pacing electrode calcification. The resulting high impedances may compromise defibrillation and pacing therapy."‡
-
When coil impedances rise above the 70/90 ohm normal range, there is increased potential for abnormal pulse widths. Abnormal pulse widths signal that the lead may not perform as expected.
Though Medtronic devices will not truncate therapy due to an abnormal pulse width in any polarity, ePTFE leads with rising impedances should be considered at risk for ineffective therapy.
*Boston Scientific, Management of Potentially Calcified ePTFE Defibrillation Lead Coil(s) Using 28-Day Averaged LVSI, published July 2025
†Koneru, Jayanthi N. et al. PO-06-203 Implantable Cardioverter Defibrillator Failure Due to High-Voltage Impedance Abnormality Causing Monophasic Shocks and Defibrillation Failure. Heart Rhythm, Volume 22, Issue 4, S722 - S723.
‡Hauser, Robert G., et al. High shocking and pacing impedances due to defibrillation lead calcification. Journal of Interventional Cardiac Electrophysiology, (2020) 58:253–259, Published online: 18 December 2019
This communication is not associated with specific models
Methods for Estimating
Contact Us
Worldwide Contact Information